
SL Paper 3
Lanthanum, La, and antimony, Sb, form compounds with bromine that have similar formulas, LaBr3 and SbBr3.
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
Markscheme
polar covalent
average electronegativity «= (3.0 + 2.0)» = 2.5 AND electronegativity difference «= 3.0 – 2.0» = 1.0
[2 marks]
ionic bonding
OR
electrostatic forces between ions
«slight» movement brings ions of same charge adjacent to each other «causing the crystal to break»
OR
«slight» movement results in repulsion between layers «causing the crystal to break»
[2 marks]
Examiners report
In order to determine the oil content of different types of potato crisps (chips), a student weighed of crushed crisps and mixed them with of non-polar solvent.
She assumed all the oil in the crisps dissolved in the solvent.
The student then filtered the mixture to remove any solids, and gently heated the solution on a hot plate to evaporate the solvent.
She measured the mass of the oil that remained from each type of crisps
Suggest why a non-polar solvent was needed.
State one reason why the mixture was not heated strongly.
Non-polar solvents can be toxic. Suggest a modification to the experiment which allows the evaporated solvent to be collected.
Suggest one source of error in the experiment, excluding faulty apparatus and human error, that would lead to the following:
Markscheme
oil is non-polar «and dissolves best in non-polar solvents»
OR
oil does not dissolve in polar solvents ✔
Do not accept “like dissolves like” only.
solvent/oil is flammable
OR
solvent/oil must be kept below its flash point
OR
oxidation/decomposition of oil
OR
mixture has a low boiling point ✔
Accept “to prevent evaporation of oil”.
distillation «instead of evaporation» ✔
Accept “pass vapour through a condenser and collect liquid”.
Do not accept “condensation” without experimental details.
Experimental mass greater than actual mass of oil in crisps:
other substances «in the crisps» are soluble in the solvent
OR
not all the solvent evaporates ✔
Experimental mass less than actual mass of oil in crisps:
not all oil dissolved/extracted ✔
Accept “oil evaporated” OR “oil burned/decomposed” OR “oil absorbed by the filter” OR “assumption «all oil dissolved» was wrong” for M2.
Do not accept examples of human errors OR faulty apparatus.
Examiners report
A well answered question where replies used all the alternatives provided. Very few candidates limited their answer to "like dissolves like" and while this expression was used most student elaborated with higher quality answer. Some common incorrect responses included students talking about dissolving the crisps (chips) or indicating the oil was a polar compound.
Another correctly answered question. As accepted by notes many candidates scored by stating "to prevent evaporation of oil". This resulted in the same argument scoring twice as often used for 1d as well. Some students incorrectly indicated the problem was to prevent the evaporation of the solvent which was the point of this step in the experiment. This could indicate a general lack of understanding of experimental methods.
A bit disappointing as the number of correct answers were substantially lower than expected. Many students responded using a fume hood or other method to remove the solvent. Once again this indicates a general misunderstanding about experimental methods.
Even weak candidates scored at least one point and often both. One common pitfall was to invert the arguments or provide answers excluded by the stem. A frequent incorrect answer was identification of faulty apparatus and human error which was specifically excluded in the question.
The mild analgesic aspirin can be prepared in the laboratory from salicylic acid.
(CH3CO)2O + HOC6H4COOH → CH3CO2C6H4COOH + CH3COOH
Salicylic acid Aspirin
After the reaction is complete, the product is isolated, recrystallized, tested for purity and the experimental yield is measured. A student’s results in a single trial are as follows.
Literature melting point data: aspirin = 138–140 °C
Determine the percentage experimental yield of the product after recrystallization. The molar masses are as follows: M(salicylic acid) = 138.13 g mol−1, M(aspirin) = 180.17 g mol−1. (You do not need to process the uncertainties in the calculation.)
Suggest why isolation of the crude product involved the addition of ice-cold water.
Justify the conclusion that recrystallization increased the purity of the product, by reference to two differences between the melting point data of the crude and recrystallized products.
State why aspirin is described as a mild analgesic with reference to its site of action.
Markscheme
ALTERNATIVE 1:
«theoretical yield = × 180.17 g mol−1 =» 2.024 «g»
«experimental yield = × 100 =» 55.53 «%»
ALTERNATIVE 2:
«»= 0.01124 «mol salicylic acid/aspirin theoretical» AND
«»= 0.006239 «mol aspirin experimental»
«experimental yield = x 100 =» 55.51 «%»
Accept answers in the range 55.4 % to 55.7 %.
Award [2] for correct final answer.
low temperature gives greater difference between solubility of aspirin and impurities
OR
«product» crystallizes out from cold solution/«ice-cold water/lower temperature» speeds up crystallization process
OR
aspirin/product has low solubility «in water» at low temperatures
intercepts pain stimulus at source/acts at site of pain
OR
interferes with production of pain sensitizing substances/prostaglandins «at site of pain»
Examiners report
recrystallized melting point is higher
OR
recrystallized melting point is closer to pure substance/literature value
smaller range of values
Polymers are made up of repeating monomer units which can be manipulated in various ways to give structures with desired properties.
(i) Draw the structure of 2-methylpropene.
(ii) Deduce the repeating unit of poly(2-methylpropene).
Deduce the percentage atom economy for polymerization of 2-methylpropene.
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
Markscheme
i
OR
H2C=C(CH3)2
ii
OR
−CH2C(CH3)2−
Continuation bonds needed for mark.
No penalty if square brackets present or “n” appears after the bracket/formula.
«same mass of product as reactant, thus» 100«%»
Accept “less than 100%” only if a reason is given (eg, the catalyst is not converted into the product, or other reasonable answer).
i
due to stability of plastics/strong covalent bonds
OR
low volatility preventing good mixing with oxygen «gas»
OR
lack of/insufficient oxygen
OR
plastics are often parts of devices with non-combustible components «which mechanically prevent the combustion of plastic components»
OR
PVC already partly oxidised «because some C–H bonds are replaced with C–Cl bonds», so it cannot produce enough heat for complete combustion
OR
many industrial/household materials contain additives that reduce their flammability/act as flame retardants
ii
weakly bound to the PVC/no covalent bonds to PVC/only London/dispersion/instantaneous induced dipole-induced dipole forces between DEHP and PVC AND leach/evaporate «from PVC» to atmosphere/food chain
OR
has low polarity/contains non-polar hydrocarbon chains AND fat-soluble/deposits in the fatty tissues
OR
has unusual structural fragments/is a xenobiotic/difficult to metabolise AND stays in the body for a long time
Examiners report
The table summarizes some properties of graphite and graphene.
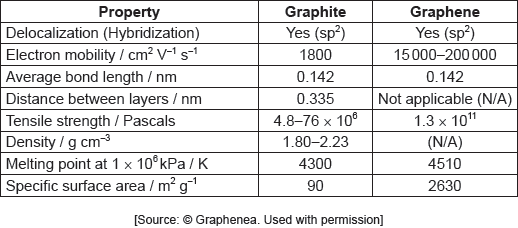
Graphene is two-dimensional, rather than three-dimensional, material.
Justify this by using the structure of graphene and information from the table.
Show that graphene is over 1600 times stronger than graphite.
Identify a value from the table which can be used to support the information about graphene given below.
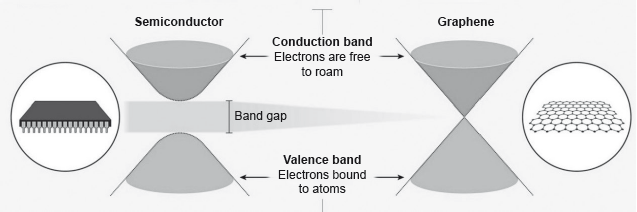
Electrons in a solid are restricted to certain ranges, or bands, of energy (vertical axis). In an insulator or semiconductor, an electron bound to an atom can break free only if it gets enough energy from heat or a passing photon to jump the “band gap”, but in graphene the gap is infinitely small.

Diamond, graphene, and graphite are all network solids.
Suggest, giving a reason, the electron mobility of diamond compared to graphene.
The melting point of diamond at 1 × 106 kPa is 4200 K (in the absence of oxygen).
Suggest, based on molecular structure, why graphene has a higher melting point under these conditions.
Markscheme
consists of single/one sheet/layer «of carbon atoms»
graphene has no density measurement
OR
graphene has no distance between layers data
OR
graphene has large specific surface area «compared to graphite»
Do not accept “sp2” alone without reference to single/one sheet/layer.
Accept “thickness of one atom” OR “consists of a plane” for M1.
[2 marks]
Any one of these alternatives:
ALTERNATIVE 1
«»
1.7 × 103/1711
ALTERNATIVE 2
1600 × 76 × 106 = 1.2 × 1011 «is less than tensile strength of graphene»
ALTERNATIVE 3
= 8.1 × 107 «is greater than upper end of tensile strength for graphite»
Accept any value in the range 1700–27 083. Answer may be expressed in scientific notation or otherwise.
Accept any value calculated which is less than the graphene tensile strength based on a value chosen from within the 4.8–76 × 106 range.
[1 mark]
«graphene has a high electron mobility of» 15 000–200 000 «cm2 V–1 s–1»
A specific value or range of values must be given.
Accept any value in the 15 000–200 000 «cm2 V–1 s–1» range.
[1 mark]
smaller/zero
no delocalized electrons/electrons are bound/electrons not free to move/electrons not free to roam
OR
localized electrons «in sigma bonds»
OR
large band gap
Accept “diamond is a dielectric” OR “diamond does not conduct electricity” for M2.
Award [1 max] for just “immobile/less mobile”.
Award [2] for “electrons immobile «in diamond» due to the large band gap” OR "electrons «in diamond» immobile
since electrons are localized «in the sigma bonds»”.
[2 marks]
shorter bonds in graphene
OR
bonds in graphene intermediate between single and double
OR
bond order in graphene is 1.33
OR
delocalization creates stronger bonds
OR
shorter bonds are stronger
stronger/shorter bonds require higher temperature/faster thermal motion to be altered
OR
stronger/shorter bonds require greater energy to be broken
[2 marks]
Examiners report
The structure of aspirin is shown in section 37 of the data booklet.
Suggest one reactant used to prepare aspirin from salicylic acid.
Aspirin, C6H4(OCOCH3)COOH, is only slightly soluble in water.
Outline, including an equation, how aspirin can be made more water-soluble. Use section 37 in the data booklet.
Markscheme
NOTE: Accept condensed structural formulas.
Accept “ethanoic acid/acetic acid/CH3COOH”.
Accept “C4H6O3” OR “C2H3OCl”.
react with sodium hydroxide/NaOH/«strong» base
OR
convert to «ionic» salt ✔
NOTE: Accept other suitable bases (eg, KOH/NaHCO3/Na2CO3) with corresponding equation for chosen base for M2.
Accept “CaCO3”, although calcium salicylate is not water soluble.
Accept ionic equation.
C6H4(OCOCH3)COOH (s) + NaOH (aq) → C6H4(OCOCH3)COONa (aq) + H2O (l) ✔
NOTE: Award [2] for M2.
Examiners report
Lactose is a disaccharide formed by the condensation reaction of the monosaccharides galactose and glucose.
Describe what is meant by a condensation reaction.
Draw the structure of galactose on the skeleton provided.
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
Markscheme
«reaction in which» two reactants/molecules/functional groups bond/react «to form a larger molecule/single main product»
small/tiny molecule
OR
H2O formed
Accept formula or name of a specified small molecule other than water such as ammonia, ethanoic/acetic acid,
ethanol, hydrogen sulfide etc. for M2.
Do not accept just “molecule formed”.
Award [1 max] for an example giving an equation of a condensation reaction such as the formation of a disaccharide.
Accept “alpha” or “beta” form of galactose.
Any two of:
makes the plastic more hydrophilic/water soluble
carbohydrates are broken down/hydrolysed by bacteria/microorganisms
makes plastic more accessible to bacteria as holes/channels are created
OR
plastic of lower density is more permeable/susceptible to water/oxygen/heat/pressure
weakens intermolecular/London/dispersion/instantaneous induced dipole-induced dipole forces «between polymer chains in the plastic»
Accept “van der Waals/vdW” for “London” forces.
[Max 2 Marks]
Examiners report
Lipids are an important part of the human diet.
Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed in this reaction and the name of the other product.
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
Markscheme
Name of the chemical link: ester/ethoxycarbonyl
AND
Name of the other product: water
Do not accept formulas.
Do not accept “esterification”
i
coconut oil AND lowest «percentage of» unsaturated fatty acids
OR
coconut oil AND smallest number of C=C bonds
OR
coconut oil AND highest «percentage of» saturated fatty acids
Accept “fats” for “fatty acids”.
ii
soybean oil AND highest «percentage of» polyunsaturated fatty acids
OR
soybean oil AND greatest number of C=C bonds
OR
soybean oil AND lowest «percentage of» saturated fatty acids
Accept “fats” for “fatty acids”.
iii
Beef fat: «P/S = = » 0.05
AND
Soybean oil: «P/S = =» 4.1
iv
«higher proportion of» polyunsaturated fatty acids decrease risk of atherosclerosis/heart disease/cardiovascular disease/CVD
OR
«higher proportion of» polyunsaturated fatty acids which are less likely to be deposited on the walls of arteries «than saturated fatty acids»
Accept converse arguments.
Accept correct arguments in terms of HDL and LDL but not in terms of “good” and “bad” cholesterol.
Accept “fats” for “fatty acids”.
v
Any two of:
cotton seed oil has «a higher proportion of» longer chain/greater molar mass fatty acids
molecules of cotton seed oil have greater surface area/have higher electron density
Accept “molecules of cotton seed oil are packed more closely/have more regular structure” for M2.
stronger London/dispersion/instantaneous induced dipole-induced dipole forces between chains in cotton seed oil
Accept converse arguments.
Accept “fats” for “fatty acids”.
Examiners report
Proteins are polymers of amino acids. A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
© International Baccalaureate Organization 2020.
Determine the value of A1.
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the α-helix in the secondary structure.
Proteins are polymers of amino acids.
Describe how the tertiary structure differs from the quaternary structure in hemoglobin.
Markscheme
✔
Accept any value within the range “”.
Ile AND larger Rf ✔
more soluble in non-polar solvent «mobile phase»
OR
not as attracted to polar «stationary» phase ✔
Only award M2 if Ile is identified in M1.
hydrogen/ bonding «between amido hydrogen and carboxyl oxygen atoms» ✔
tertiary: folding/shape of a single «polypeptide/protein» chain ✔
quaternary: arrangement/folding of four/several chains/proteins/polypeptides «held together by » ✔
Accept “two or more polypeptides” for M2.
Examiners report
Many students scored this mark. The students who missed this mark that were close either measured from the top or bottom of the spot rather than the middle. A few students had answers that were greater than 1 which indicated a clear lack of understanding of this concept.
Not well answered. Many candidates referred to glycine even when they had obtained the correct Rf value in 5a. Answers referring to Molar mass and isoelectric point were quite common. Some candidates that identified Ile correctly lost the first mark as didn't make any reference to the Rf. There was a clear lack of understanding that the Rf value was related to polarity not molar mass.
A well answered question.
Many candidates didn't understand the question and provided long answers referring to the interactions but failing to identify those took place within the same/one chain. More candidates were able to score the second mark referring to multiple chains.
Polypropene is used to make many objects including carpets, stationery and laboratory equipment.
Draw a section of an isotactic polypropene polymer chain containing four repeating units.
Predict, with a reason, whether isotactic or atactic polypropene has the higher melting point.
Polypropene is a thermoplastic. Outline what is meant by thermoplastic.
Discuss why the recycling of plastics is an energy intensive process.
Markscheme
NOTE: Continuation bonds must be shown.
Ignore square brackets and “n”.
Do not accept one repeating unit in square brackets with a subscript of 4.
Accept condensed structure provided all C to C bonds are shown and CH3 groups on same side.
Accept
Do not accept syndiotactic (alternating orientation of the CH3 groups).
isotactic «has higher melting point» AND ordered chains pack more closely
OR
isotactic «has higher melting point» AND stronger intermolecular/London/dispersion forces ✔
NOTE: Accept “van der Waals’ forces”/”vdW”.
softens when heated «and hardens when cooled» ✔
Any two of:
collection/transportation of plastic waste ✔
separation of different types «of plastic»
OR
separation of plastic from other materials ✔
melting plastic ✔
processing/washing/cleaning/drying/manufacture of recycled plastic ✔
Examiners report
Aspirin is formed by reacting salicylic acid with ethanoic anhydride. The structure of aspirin is given in section 37 of the data booklet.
Deduce the structural formula of the by-product of this reaction.
Aspirin crystals are rinsed with water after recrystallization to remove impurities.
Suggest why cold water is used.
The solubility of aspirin is increased by converting it to an ionic form. Draw the structure of the ionic form of aspirin.
Comment on the risk of overdose when taking aspirin as an analgesic, referring to the following values, for a person weighing :
Minimum therapeutic dose
Estimated minimum lethal dose
Markscheme
OR
✔
Accept full OR condensed structural formula.
to avoid dissolving the crystals/aspirin ✔
Accept “to avoid loss of product” OR “aspirin is less soluble in cold water”.
✔
Accept a positive metal ion next to the such as “”.
Accept “” without showing the charges.
Accept notations such as “” OR “” OR “” but not “” OR “” OR “”.
low/medium risk «of overdosing» AND «estimated» lethal dose is times/much larger than therapeutic dose
OR
times the dose results in chance of dying ✔
Accept “ and low/medium risk due to large therapeutic index”.
Do not accept “low/medium risk AND large therapeutic window”.
Do not accept “ times the dose” alone for the mark.
Examiners report
A well answered question. Most candidates chose to enter the full structure. Some incorrect answers gave the aspirin product or the salicylic acid rather than the acetic acid.
While there were many good answers it was worrying to correct as many where the student clearly didn't establish a connection between solubility and purification. Many incorrect responses indicated the cold water was to "stay below the melting point of the aspirin" rather than relate it to the solubility of the final product.
Not well answered. Students evidenced familiarity with the content but failed to provide correct structures. Showing lines used to represent covalent bonds to show an ionic interaction, using convention for complexes, showing only one ion were some of the many mistakes. Many students also had an incorrect product having the aspirin lose the -OH group from the carboxylic acid or the entire carboxylic acid functional group rather than just the H+.
This is a topic that continues to challenge students and "low/medium risk AND large therapeutic window" was worryingly common. There were also many students who did not calculate the ratio correctly.
Solubility plays an important role in the bioavailability of drugs in the body.
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
Formulate an equation for the conversion of aspirin to a more water soluble derivative.
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
Markscheme
presence of «large» benzene/arene ring AND non-polar/hydrophobic
OR
presence of «large» benzene/arene ring AND cannot form H-bond with water
contain COOH/carboxyl/–OH/hydroxyl «and ester group» AND polar/hydrophilic
OR
contain COOH/carboxyl/–OH/hydroxyl «and ester group» AND can form H-bonds with water
Accept “phenyl” for “benzene ring”.
Accept "carboxylic acid" for "carboxyl".
Do not accept "alcohol" for "hydroxyl".
[2 marks]
OR
C6H4(OCOCH3)COOH + NaOH → C6H4(OCOCH3)COONa + H2O
Charges (O– and Na+) not necessary to score the mark.
Accept net ionic equation.
Accept any strong base in place of NaOH.
[1 mark]
«student’s» sample impure
lattice disrupted/not uniform «due to presence of impurities»
OR
fewer interparticle/intermolecular forces «due to presence of impurities»
Accept converse arguments.
[2 marks]
One similarity:
peak at 2500–3000 «cm–1»/peak due to O–H/hydroxyl in carboxylic acids
OR
peak at 1700–1750 «cm–1»/peak due to C=O/carbonyl
OR
peak at 2850–3090 «cm–1»/peak due to C–H of arene
One difference:
peak at 3200–3600 «cm–1» in salicylic acid/ peak due to O–H in phenol in salicylic acid
OR
«two» peaks at 1700–1750 «cm–1» in aspirin AND one peak «in the same area» in salicylic acid
Accept “peak at 1600 cm–1 for arene/benzene ring” – not in the data booklet.
Accept “2500–3600 cm–1 «overlapping absorptions of two O–H» in salicylic acid”.
Accept “stronger/broader/split peak at 1700–1750 cm–1 in aspirin”.
[2 marks]
«use of» alternative solvents such as supercritical/liquid CO2
OR
use of water «as solvent»
OR
solvent-free reactions «for example, polymerization of propene»
OR
solid-state chemistry
OR
recycle «waste» solvents
OR
catalysis that leads to better/higher yield
OR
reducing number of steps
Do not accept political/regulatory solutions.
“catalysis” not sufficient for mark.
[1 mark]
Examiners report
Palmitic acid has a molar mass of 256.5 g mol−1.
The apparatus in the diagram measures the surface pressure created by palmitic acid molecules on the surface of water. This pressure is caused by palmitic acid molecules colliding with the fixed barrier. The pressure increases as the area, A, available to the palmitic acid is reduced by the movable barrier.
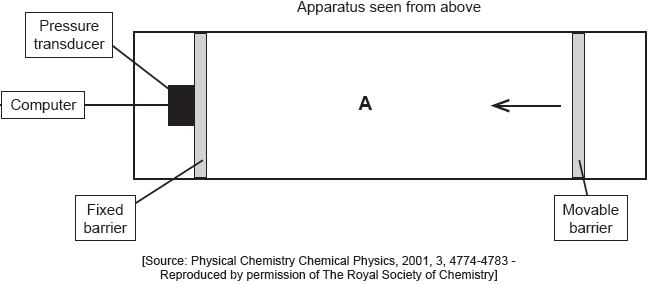
When a drop of a solution of palmitic acid in a volatile solvent is placed between the barriers, the solvent evaporates leaving a surface layer. The graph of pressure against area was obtained as the area A was reduced.
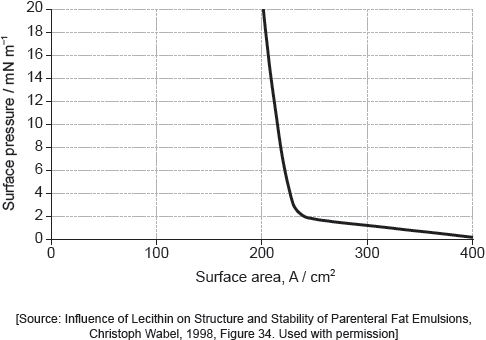
Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
Suggest why there is a small increase in the surface pressure as the area is reduced to about 240 cm2, but a much faster increase when it is further reduced.
The solution of palmitic acid had a concentration of 0.0034 mol dm−3. Calculate the number of molecules of palmitic acid present in the 0.050 cm3 drop, using section 2 of the data booklet.
Assuming the sudden change in gradient occurs at 240 cm2, calculate the area, in cm2, that a single molecule of palmitic acid occupies on surface of the water.
If you did not obtain an answer for (b)(ii) use a value of 8.2 × 1016, but this is not the correct answer.
Markscheme
Must cut CH2–CO bond AND enclose all of the –COOH group.
[1 mark]
Any two of:
–COOH/CO/OH/carboxylate/carboxyl/hydroxyl/hydroxy group forms hydrogen bonds/H-bonds to water
London/dispersion/instantaneous induced dipole-induced dipole forces occur between hydrocarbon chains
hydrocarbon chain cannot form hydrogen bonds/H-bonds to water
strong hydrogen bonds/H-bonds between water molecules exclude hydrocarbon chains «from the body of the water»
Accept “hydrophilic part/group forms hydrogen bonds/H-bonds to water”.
Accept “hydrophobic section” instead of “hydrocarbon chain”.
Award [1 max] for answers based on “the –COOH group being polar AND the hydrocarbon chain being non-polar”.
[2 marks]
Above about 240 cm2:
greater collision frequency/collisions per second between «palmitic acid» molecules and the barrier «as area reduced»
At less than about 240 cm2:
molecules completely cover the surface
OR
there is no space between molecules
OR
force from movable barrier transmitted directly through the molecules to the fixed barrier
OR
«palmitic acid» molecules are pushed up/down/out of layer
For both M1 and M2 accept “particles” for “molecules”.
For M1 accept “space/area between molecules reduced” OR “molecules moving closer together”.
[2 marks]
amount of acid = «5.0 × 10–5 dm3 × 0.0034 mol dm–3» = 1.7 × 10–7 «mol»
number of molecules = «1.7 × 10–7 mol × 6.02 × 1023 mol–1 =» 1.0 × 1017
Award [2] for correct final answer.
Award [1] for “1.0 × 1020”.
[2 marks]
«area = » 2.4 × 10–15 «cm2»
[1 mark]
Examiners report
The development of materials with unique properties is critical to advances in industry.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
Markscheme
Any two of:
ability to form a LC phase
chemically stable
«LC phase that is» stable over suitable temperature range
polar
OR
being able to change orientation with applied electric field
rapid switching speed «responds to changes of voltage quickly»
Accept “ability of molecules to transmit light under certain conditions” OR “rodshaped molecules” OR “stable to light/not light sensitive”.
[Max 2 Marks]
branching in LDPE prevents close packing «of chains»
LDPE is more flexible/less rigid
OR
LDPE has lower «tensile» strength
Do not accept “difference in density”.
Award [1 max] for stating “branching in LDPE AND little/no branching in HDPE”.
B AND absence «of absorption of» C–H at 2850–3090 «cm–1»
OR
B AND presence of «absorption of» C–F at 1000–1400 «cm–1»
(–C2H3Cl–)2 (s) + 5O2 (g) → 4CO2 (g) + 2H2O (l) + 2HCl (g)
correct species in reactants and products
balanced
Accept “(–C2H3Cl–)2 (s) + 5.5O2 (g) → 4CO2 (g) + 3H2O (l) + Cl2 (g)”.
Award M2 only if M1 correct.
Examiners report
Sunflower oil contains stearic, oleic and linoleic fatty acids. The structural formulas of these acids are given in section 34 of the data booklet.
Explain which one of these fatty acids has the highest boiling point.
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 moldm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
Markscheme
stearic acid AND chain has no kinks/more regular structure
OR
stearic acid AND it has straight chain
OR
stearic acid AND no C=C/carbon to carbon double bonds
OR
stearic acid AND saturated
OR
stearic acid AND chains pack more closely together
stronger London/dispersion/instantaneous induced dipole-induced dipole forces «between molecules»
Accept “stearic acid AND greater surface area/electron density”.
M2 can only be scored if stearic acid is correctly identified.
Accept “stronger intermolecular/van der Waals’/vdW forces”.
[2 marks]
«n(I2) = 0.123 dm3 x 0.500 moldm–3 =» 0.0615 «mol»
«m(I2) = 0.0615 mol x 253.8 gmol–1 =» 15.6 «g»
«iodine number » = 156
Award [3] for correct final answer.
Iodine number must be a whole number.
Award [2 max] for 78.
[3 marks]
Examiners report
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
Markscheme
«mostly» non-polar
OR
hydrocarbon backbone
OR
only 1 hydroxyl «group so mostly non-polar»
Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
[1 mark]
Examiners report
There has been significant growth in the use of carbon nanotubes, CNT.
Explain these properties of carbon nanotubes.
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
Pure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
© International Baccalaureate Organization 2020.
Write the half-equations for the reactions occurring in this electrolysis.
Calculate the theoretical mass of magnesium obtained if a current of is used for hours. Use charge and section 2 of the data booklet
Suggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their structure, the high selectivity of zeolites.
Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how CNT molecules could be classified as nematic.
Markscheme
Excellent strength: defect-free AND rigid/regular 2D/3D ✔
Excellent conductivity: delocalized electrons ✔
Accept “carbons/atoms are all covalently bonded to each other” for M1.
Any of:
ductility ✔
strength/resistance to deformation ✔
malleability ✔
hardness ✔
resistance to corrosion/chemical resistance ✔
range of working temperatures ✔
density ✔
Do not accept “conductivity”.
Anode: ✔
Cathode: ✔
Accept .
Award [1 max] for correct equations at incorrect electrodes.
✔
✔
✔
Award [3] for correct final answer.
argon//helium/ ✔
Accept any identified noble/inert gas.
Accept name OR formula.
Do not accept “nitrogen/“.
pores/cavities/channels/holes/cage-like structures ✔
«only» reactants with appropriate/specific size/geometry/structure fit inside/go through/are activated/can react ✔
Accept “molecules/ions” for “reactants” in M2.
rod-shaped molecules
OR
«randomly distributed but» generally align
OR
no positional order AND have «some» directional order/pattern ✔
Accept “linear” for “rod-shaped”.
Examiners report
This was a very popular option with approximately 34% of candidates attempting Option B. Many students appeared well prepared for this option. Some candidates continue to provide answers with a heavy Biology bias that often make them lose valuable points.
This was a very popular option with approximately 34% of candidates attempting Option B. Many students appeared well prepared for this option. Some candidates continue to provide answers with a heavy Biology bias that often make them lose valuable points.
This was a very popular option with approximately 34% of candidates attempting Option B. Many students appeared well prepared for this option. Some candidates continue to provide answers with a heavy Biology bias that often make them lose valuable points.
This was a very popular option with approximately 34% of candidates attempting Option B. Many students appeared well prepared for this option. Some candidates continue to provide answers with a heavy Biology bias that often make them lose valuable points.
This was a very popular option with approximately 34% of candidates attempting Option B. Many students appeared well prepared for this option. Some candidates continue to provide answers with a heavy Biology bias that often make them lose valuable points.
This was a very popular option with approximately 34% of candidates attempting Option B. Many students appeared well prepared for this option. Some candidates continue to provide answers with a heavy Biology bias that often make them lose valuable points.
This was a very popular option with approximately 34% of candidates attempting Option B. Many students appeared well prepared for this option. Some candidates continue to provide answers with a heavy Biology bias that often make them lose valuable points.
Peptidase enzyme in the digestive system hydrolyses peptide bonds.
A tripeptide Ala-Asp-Lys was hydrolysed and electrophoresis of the mixture of the amino acids was carried out at a pH of 6.0. Refer to section 33 of the data booklet.
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
Identify the name of the amino acid that does not move under the influence of the applied voltage.
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
Outline how lead ions could be removed from an individual suffering from lead poisoning.
Markscheme
catabolism/catabolic
[1 mark]
alanine
Do not accept ala.
[1 mark]
Lys/lysine
pH «buffer» < pI «Lys»
OR
buffer more acidic than Lys «at isoelectric point»
OR
«Lys» exists as
OR
«Lys» charged positively/has +1/1+ «overall» charge «and moves to negative electrode»
Do not apply ECF from M1.
Accept converse argument.
Do not accept just “has H3N+ group” for M2 (as H3N+ is also present in zwitterion).
Do not penalize if COOH is given in the structure of lysine at pH 6 instead of COO–.
[2 marks]
highest frequency of successful collisions between active site and substrate
OR
highest frequency of collisions between active site and substrate with sufficient energy/ AND correct orientation/conformation
OR
optimal shape/conformation of the active site «that matches the substrate»
OR
best ability of the active site to bind «to the substrate»
Accept “number of collisions per unit time” for “frequency”.
Do not accept “all active sites are occupied”.
[1 mark]
ascorbic acid/vitamin C
[1 mark]
react/bind/chelate with enzyme
OR
disrupt ionic salt bridges
OR
affect shape of tertiary/quaternary structures
OR
precipitate enzymes
OR
break/disrupt disulfide bridges/bonds
Do not accept “changes shape of active site” by itself.
[1 mark]
«use of» host-guest chemistry
OR
chelation «therapy»
Accept specific medication/chelating agent such as EDTA, CaNa2 EDTA, succimer, D-penicillamine, dimercaprol.
[1 mark]
Examiners report
| Phospholipids are a main component of cell membranes. |
Deduce the products of the hydrolysis of a non-substituted phospholipid, where and represent long alkyl chains.
A representation of a phospholipid bilayer cell membrane is shown:
© International Baccalaureate Organization 2020.
Identify the components of the phospholipid labelled A and B.
State the most significant intermolecular forces in the phospholipid in b(i).
Phospholipids help maintain cellular environments while fatty acid lipids have important roles in energy storage and electrical insulation. Discuss the structural properties of saturated fats needed for these roles.
Markscheme
glycerol ✔
both fatty acids AND phosphoric acid ✔
Accept either names OR structures.
Accept “long chain carboxylic acid” for “fatty acid”.
Penalize once only if an incorrect name is given for a correct structure or vice-versa.
A: phosphate/ionic group
AND
B: alkyl/hydrocarbon «chain» ✔
Accept "glycerol «fragment»" OR "glycerophosphate" OR “ester” for A.
Accept “fatty acid «tail»” for B.
Do not accept terms such as “polar head”, “non-polar tail”, “hydrophilic” OR “hydrophobic” for components alone.
Forces occurring between components labelled A:
hydrogen/ bonding
OR
ion–dipole
OR
ionic/electrostatic «repulsion and/or attraction» ✔
Accept “dipole-dipole” for M1.
Do not accept “van der Waals/vdW” for M1.
Forces occurring between components labelled B:
dispersion/London/instantaneous dipoles/temporary dipoles ✔
Accept “van der Waals/vdW” for M2.
Energy storage:
not water-soluble/no hydrogen/ bonding
OR
less oxidized/more reduced
OR
high energy stored in bonds
OR
high «negative» enthalpy of combustion/oxidation ✔
Accept “potential energy” for “stored energy”.
Electrical insulator:
no delocalized electrons/conjugation ✔
Examiners report
Not as well answered as expected. However, many candidates managed to score at least one point. Quite a few lost the only mark due to wrong linkage. Some students drew fatty acid structures with aldehydes or phosphoric acid with incorrect bond linkages in the structure (OH-).
Mostly well answered. Weaker candidates used the terms hydrophobic (non-polar) tail and/or hydrophilic (polar) head and therefore lost the mark. Students are expected to know the names of these structures.
Many good answers. Those that failed to score often provided the inverted answer. Dipole-dipole was fairly common for M1 but VdW forces less so for M2.
Not well answered. In particular the first mark seemed particularly challenging for students. Even when at times wording wasn't enough to allow BOD for M2 it was evident the candidate had some idea but none for the first one. Non-polar allowed many students to score the second mark.
Materials science involves understanding the properties of materials and applying those properties to desired structures.
Magnesium oxide, MgO, and silicon carbide, SiC, are examples of ceramic materials. State the name of the predominant type of bonding in each material.
Predict the predominant type of bonding for a binary compound AB in which the electronegativity of both atoms is low. Use section 29 of the data booklet.
Markscheme
MgO: ionic AND SiC: covalent
Accept “covalent network/network covalent” for “covalent” but not just “network”.
metallic «bonding»
Examiners report
Vitamins are organic compounds essential in small amounts.
State the name of one functional group common to all three vitamins shown in section 35 of the data booklet.
Explain the biomagnification of the pesticide DDT.
Explain why maltose, C12H22O11, is soluble in water.
Markscheme
hydroxyl ✔
NOTE: Accept “hydroxy” but not “hydroxide”.
Accept “alkenyl”.
Do not accept formula.
accumulates in fat/tissues/living organisms
OR
cannot be metabolized/does not break down «in living organisms»
OR
not excreted / excreted «very» slowly ✔
passes «unchanged» up the food chain
OR
increased concentration as one species feeds on another «up the food chain» ✔
NOTE: Accept “lipids” for “fat”.
«solubility depends on forming many» H-bonds with water ✔
maltose has many hydroxyl/OH/oxygen atom/O «and forms many H-bonds» ✔
NOTE: Reference to “with water” required.
Accept “hydroxy” for “hydroxyl” but not “hydroxide/OH–”.
Reference to many/several OH groups/O atoms required for M2.
Examiners report
Infrared (IR) spectroscopy is often used for the identification of polymers, such as PETE, for recycling.
LDPE and high density polyethene (HDPE) have very similar IR spectra even though they have rather different structures and physical properties.
Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density polyethene (LDPE).
Deduce, giving your reasons, the identity and resin identification code (RIC) of A and B using sections 26 and 30 of the data booklet.
Describe the difference in their structures.
Explain why the difference in their structures affects their melting points.
Markscheme
A RIC: 1 AND B RIC: 4
ALTERNATIVE 1:
«only» PETE contains carbonyl/C=O/ester/COO groups
carbonyl groups absorb at 1700–1750 «cm–1»
ALTERNATIVE 2:
LDPE contains more C–H bonds «than PETE»
C–H bonds absorb at 2850–3090 «cm–1»
For either, accept specific frequencies in these ranges (eg 1735 «cm–1» or 2900 «cm–1»).
[3 marks]
HDPE less branched
OR
LDPE more branched
Accept “no branching in HDPE AND branching in LDPE”.
[1 mark]
HDPE «polymer» chains/molecules can pack together more closely «than LDPE chains»
OR
HDPE «polymer» chains/molecules have a higher contact surface area «than LDPE chains»
stronger intermolecular/dispersion/London/van der Waals’ forces in HDPE AND higher melting point
Accept converse arguments.
[2 marks]
Examiners report
Lipids and carbohydrates contain the same elements but have different properties.
List the building blocks of triglycerides and carbohydrates.
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16.
Markscheme
Triglycerides:
organic acid/fatty acid and glycerol/propane-1,2,3-triol
AND
Carbohydrates:
monosaccharides
Accept simple sugars.
[1 mark]
«water/aqueous solubility depends on forming many» H-bonds with water
raffinose has many hydroxyl/O–H/oxygen atoms/O «and forms many H-bonds» AND linoleic acid has few/one hydroxyl/O–H/oxygen atom/O/carboxyl group/ COOH/is largely non-polar «and cannot form many H-bonds»
Accept statement which implies comparison.
[2 marks]
«base» hydrolysis/saponification
OR
«produces glycerol and» soap/salt of the «fatty» acid
«products are» water soluble «and drain away»
Accept condensed formulas.
Accept non-balanced equation.
Accept “RCOONa”.
[2 marks]
linoleic acid/C18H32O2 combustion/oxidation more exothermic «per mol»
linoleic acid/C18H32O2 has lower proportion/number of O atoms
OR
linoleic acid/C18H32O2 is less oxidized
Accept converse arguments.
[2 marks]
Examiners report
Metals are extracted from their ores by various means.
Aluminium is produced by the electrolysis of alumina (aluminium oxide) dissolved in cryolite.
Discuss why different methods of reduction are needed to extract metals.
Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
Write half-equations for the electrolysis of molten alumina using graphite electrodes, deducing the state symbols of the products.
Anode (positive electrode):
Cathode (negative electrode):
Markscheme
ions of more reactive metals are harder to reduce
OR
more reactive metals have more negative electrode potentials ✔
electrolysis is needed/used for most reactive metals
OR
carbon is used to reduce metal oxides of intermediate reactivity/less reactive than carbon
OR
heating ore is sufficient for less reactive metals ✔
NOTE: Award [1 max] for “«ease of reduction/extraction» depends on reactivity”.
electronegativity difference = 1.8 «and average electronegativity = 2.5» ✔
57 «%» ✔
NOTE: Accept any value in the range 52−65 %.
Award [2] for correct final answer.
Anode (positive electrode):
2O2− → 4e− + O2(g)
OR
2O2− + C → 4e− + CO2 (g) ✔
NOTE: Award [1 max] for M1 and M2 if correct half-equations are given at the wrong electrodes OR if incorrect reversed half-equations are given at the correct electrodes.
Cathode (negative electrode):
Al3+ + 3e− → Al (l) ✔
O2 gas AND Al liquid ✔
NOTE: Only state symbols of products required, which might be written as (g) and (l) in half-equations. Ignore any incorrect or missing state symbols for reactants.
Examiners report
The structures of morphine, diamorphine and codeine are given in section 37 of the data booklet.
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
Suggest a reagent used to prepare diamorphine from morphine.
Suggest one reason why codeine is available without prescription in some countries whilst morphine is administered under strict medical supervision.
Markscheme
Any two of:
diamorphine has ester/ethanoate/acetate «groups» AND morphine has hydroxyl «groups»
diamorphine/ester/ethanoate/acetate groups less polar
diamorphine more soluble in lipids
Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
Accept “diamorphine non-polar”.
Accept converse statements.
[2 marks]
ethanoic/acetic anhydride
OR
ethanoyl/acetyl chloride
Accept other possible reagents, such as ethanoic/acetic acid or acetyl bromide.
Accept chemical formulas.
[1 mark]
morphine has a smaller therapeutic window
Accept converse statements.
Accept “codeine has lower activity” OR “codeine has lower risk of overdose” OR “codeine is less potent”.
Do not accept “lower abuse potential for codeine” OR “codeine less addictive” OR “codeine has a lower bioavailability”.
[1 mark]
Examiners report
Stearic acid (Mr = 284.47) and oleic acid (Mr = 282.46) have the same number of carbon atoms. The structures of both lipids are shown in section 34 of the data booklet.
The iodine number is the number of grams of iodine which reacts with 100 g of fat. Calculate the iodine number of oleic acid.
State one impact on health of the increase in LDL cholesterol concentration in blood.
Explain why stearic acid has a higher melting point than oleic acid.
State one similarity and one difference in composition between phospholipids and triglycerides.
Similarity:
Difference:
Identify a reagent that hydrolyses triglycerides.
Markscheme
«one C=C bond»
«1 mole iodine : 1 mole oleic acid»
« =» 89.85 «g of I2» ✔
NOTE: Accept “90 «g of I2»”.
atherosclerosis/cholesterol deposition «in artery walls»/increases risk of heart attack/stroke/cardiovascular disease/CHD ✔
NOTE: Accept “arteries become blocked/walls become thicker”, “increases blood pressure”, OR “blood clots”.
Do not accept “high cholesterol” OR "obesity"
no kinks in chain/more regular structure
OR
straight chain
OR
no C=C/carbon to carbon double bonds
OR
saturated
OR
chains pack more closely together ✔
NOTE: Accept “greater surface area/electron density” for M1.
stronger London/dispersion/instantaneous induced dipole-induced dipole forces «between molecules» ✔
NOTE: Accept “stronger intermolecular/van der Waals’/vdW forces” for M2.
Similarity:
«derived from» propane-1,2,3-triol/glycerol/glycerin/glycerine
OR
«derived from» at least two fatty acids
OR
contains ester linkages
OR
long carbon chains ✔
NOTE: Do not accept “two fatty acids as both a similarity and a difference”.
Do not accept just “hydrocarbon/carbon chains”.
Difference:
phospholipids contain two fatty acids «condensed onto glycerol» AND triglycerides three
OR
phospholipids contain phosphate/phosphato «group»/residue of phosphoric acid AND triglycerides do not ✔
NOTE: Accept “phospholipids contain phosphorus AND triglycerides do not".
Accept “phospholipids are amphiphilic AND triglycerides are not” OR “phospholipids have hydrophobic tails and hydrophilic heads AND triglycerides do not”.
«concentrated» NaOH (aq)/sodium hydroxide
OR
«concentrated» HCl (aq)/hydrochloric acid
OR
enzymes/lipases ✔
NOTE: Accept other strong acids or bases.
Examiners report
Vitamins can be water-soluble or fat-soluble.
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
State one function of vitamin D in the body.
Markscheme
«mainly» hydrocarbon/non-polar «structure»
forms London/dispersion/instantaneous induced dipole-induced dipole forces «with fats»
Accept “forms van der Waals’/vdW forces”.
Award [1 max] for “contains only one OH/hydroxyl AND cannot form «enough» H-bonds”.
helps absorb calcium
OR
helps build bones
OR
helps keep bones healthy
OR
helps block the release of parathyroid hormone
OR
helps in muscle function
OR
helps immune system function
OR
cell growth
OR
reduction of inflammation
OR
protection from osteoporosis
OR
prevents rickets
Accept helps prevent colon/breast/prostate cancer.
Accept treat/prevent diabetes/heart disease/high blood pressure/multiple sclerosis.
Accept other correct answers.